
Chemistry, 20.09.2019 06:20 jonmorton159
Which of the following statements concerning the reaction shown below are true? p4 (s) + 3o2 (g) → p4o6 (s) ∆h = -1640 kj i. heat is absorbed ii. heat is released iii. rxn is exothermic iv. rxn is endothermic v. products have higher enthalpy content than reactants vi. reactants have higher enthalpy content than products

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 17:00, smelcher3900
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 23:00, 1315055427
Which subshell is represented by the actinides family?
Answers: 1

Chemistry, 23.06.2019 01:10, minasotpen1253
Volume is a measurement of how fast particles of a substance are moving
Answers: 3
You know the right answer?
Which of the following statements concerning the reaction shown below are true? p4 (s) + 3o2 (g) →...
Questions in other subjects:


Mathematics, 16.12.2019 03:31


Social Studies, 16.12.2019 03:31

Chemistry, 16.12.2019 03:31

Business, 16.12.2019 03:31




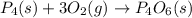
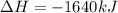
 for the reaction comes out to be negative.
for the reaction comes out to be negative.


