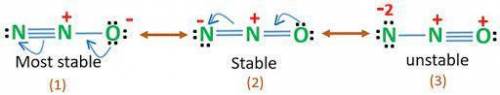
Dinitrogen monoxide has a structural formula of NNO and requires resonance structures in order to draw the Lewis structures of the molecule. Based on formal charge distributions, themostsignificant (stable) resonance structure for this molecule exhibits the order of formal charges for the 1st N, the central N, and the O atoms, respectively, as:
A. 0,+1,-1
B. -1,+1,0
C. -2,+3,-1
D. 0,0,0

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, mykalwashington
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1


Chemistry, 22.06.2019 06:10, Kianna000
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

You know the right answer?
Dinitrogen monoxide has a structural formula of NNO and requires resonance structures in order to dr...
Questions in other subjects:


Biology, 06.11.2020 01:00

English, 06.11.2020 01:00

Spanish, 06.11.2020 01:00

Biology, 06.11.2020 01:00