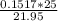Chemistry, 09.04.2020 04:37 Bearboy5957
A buret is filled with 0.1517 m naoh (aq). 25.0 ml of an unknown acid ha (aq) and two drops of indicator are added to an erlenmeyer flask, and the titration experiment is carried out. if the initial buret reading was 0.55 ml, and the buret reading at the endpoint was 22.50 ml, what is the molarity of the unknown acid, ha?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 20:30, demarcuswiseman
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
A buret is filled with 0.1517 m naoh (aq). 25.0 ml of an unknown acid ha (aq) and two drops of indic...
Questions in other subjects:


Mathematics, 06.05.2021 07:00


English, 06.05.2021 07:00






English, 06.05.2021 07:00

 where 1 and 2 are the values for acid and base respectively.
where 1 and 2 are the values for acid and base respectively.