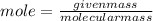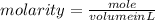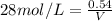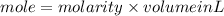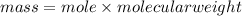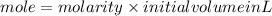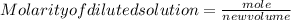1. What volume in mL of 28.0M H2SO4 is needed to contain 53.0g of H2SO4?
2. How many grams of calcium hydroxide Ca(OH)2 are needed to make 532.0 mL of a 1.90M solution?
3. If 12 mL of water are added to 124mL of a 0.505M K2SO4 solution, what will the molarity of the diluted solution be?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1

Chemistry, 22.06.2019 22:30, skinniestoflegends
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 23.06.2019 15:00, RegencySlayer5304
Charlene puts together two isosceles triangles so that they share a base, creating a kite. the legs of the triangles are 10 inches and 17 inches, respectively. if the length of the base for both triangles is 16 inches long, what is the length of the kite’s other diagonal? 6 inches inches inches 21 inchesanswer is d on e2020edit: it's geometry not chemistry, sorry.
Answers: 3
You know the right answer?
1. What volume in mL of 28.0M H2SO4 is needed to contain 53.0g of H2SO4?
2. How many gra...
2. How many gra...
Questions in other subjects:


Mathematics, 28.10.2020 01:00

Arts, 28.10.2020 01:00



Social Studies, 28.10.2020 01:00