34 atoms of Carbon (C) react with 22 molecules of Hydrogen gas (H2). How many molecules of methane (CH4) will be formed and what will be left over?
a. 17 molecules of methane formed, 5 molecules of hydrogen left over
b. 11 molecules of methane formed, 23 molecules of carbon left over
c. 5 molecules of methane formed, 29 atoms of carbon and 2 molecules of hydrogen left over
d. 22 molecules of methane formed, 12 atoms of carbon left over

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, cynthiagutierrez65
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1


Chemistry, 22.06.2019 01:20, whrjegt4jrnfdvj
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 10:30, esnyderquintero
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2
You know the right answer?
34 atoms of Carbon (C) react with 22 molecules of Hydrogen gas (H2). How many molecules of methane (...
Questions in other subjects:

Advanced Placement (AP), 16.07.2019 19:10

Spanish, 16.07.2019 19:10





Mathematics, 16.07.2019 19:10

Mathematics, 16.07.2019 19:10


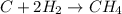
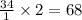 molecules of hydrogen gas.
molecules of hydrogen gas.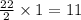 molecule of methane.
molecule of methane.