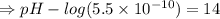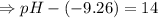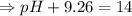Chemistry, 09.04.2020 03:05 trevorhenyan51
Calculate [H3O+] at 25 ∘C for each solution and determine if the solution is acidic, basic, or neutral. a.) [OH−] = 3.8×10−2 Mb.) [OH−] = 1.0×10-7 Mc.) [OH−] = 5.5×10−10 MPlease show the work and how you determine if the solution is neutral, acidic, or basic

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 13:00, taylorpayne525p8qxky
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 13:30, kkingstone7062
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
Calculate [H3O+] at 25 ∘C for each solution and determine if the solution is acidic, basic, or neutr...
Questions in other subjects:



History, 23.07.2019 06:30



Mathematics, 23.07.2019 06:30




![pH=-log[H_3O^+]](/tpl/images/0591/2031/6e71a.png)
![[H_3O^+][OH^-]=K_w=10^{-14}](/tpl/images/0591/2031/b8614.png) [ at 25°C]
[ at 25°C]![log[H_3O^+]+log[OH^-]=logK_w=log 10^{-14}](/tpl/images/0591/2031/bb8c2.png)
![\Rightarrow - log[H_3O^+]-log[OH^-]=-logK_w=-log 10^{-14}](/tpl/images/0591/2031/a8528.png)
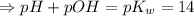
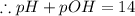
![[OH^-]=3.8\times10^{-2}M](/tpl/images/0591/2031/42d58.png)
![- log[H_3O^+]-log[OH^-]=14}](/tpl/images/0591/2031/0c29a.png)
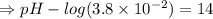
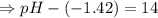
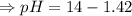
![[OH^-]=1.0\times10^{-7}M](/tpl/images/0591/2031/ac083.png)
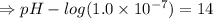
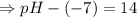
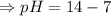
![[OH^-]=5.5\times10^{-10}M](/tpl/images/0591/2031/f6aae.png)