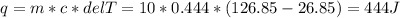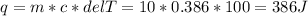Chemistry, 09.04.2020 01:32 Ashleyvasquez2261
Two 10g blocks, one of copper and one of iron, were heated from 300 K to 400K (a temperature difference of 100 K).
How much energy, in Joules, was absorbed by the iron block? *
How much energy, in Joules, was absorbed by the copper block? *

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, chrisxxxrv24
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1



Chemistry, 23.06.2019 00:30, evelynalper08
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
Two 10g blocks, one of copper and one of iron, were heated from 300 K to 400K (a temperature differe...
Questions in other subjects: