
Chemistry, 09.04.2020 00:57 ChaseRussell24
A proposed mechanism for the reaction Cl2(g) + CHCl3(g) HCl(g) + CCl4(g) in the atmosphere is Step 1: Cl2(g) 2 Cl(g) (very fast, reversible) Step 2: Cl(g) + CHCl3(g) HCl(g) + CCl3(g) (slow) Step 3: Cl(g) + CCl3(g) CCl4(g) (fast) What is the rate law for the overall reaction?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 15:30, vivianfling
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 22.06.2019 20:30, huangjianhe135
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
A proposed mechanism for the reaction Cl2(g) + CHCl3(g) HCl(g) + CCl4(g) in the atmosphere is Step...
Questions in other subjects:

Mathematics, 16.07.2019 19:00

History, 16.07.2019 19:00

Chemistry, 16.07.2019 19:00





Health, 16.07.2019 19:00


History, 16.07.2019 19:00

![\text{Rate}=k[Cl_2]^{1/2}[CCl_4][CHCl_3][CCl_3]^{-1}](/tpl/images/0590/9105/bc5d3.png)

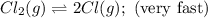
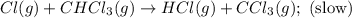

![\text{Rate}=K_2[Cl][CHCl_3]](/tpl/images/0590/9105/4079f.png) ......(1)
......(1)![K_1=\frac{[Cl]^2}{[Cl_2]}](/tpl/images/0590/9105/5c3f3.png)
![[Cl]=\sqrt{K_1[Cl_2]}](/tpl/images/0590/9105/af496.png)
![K_3=\frac{[CCl_4]}{[Cl][CCl_3]}](/tpl/images/0590/9105/b542e.png)
![[Cl]=\frac{[CCl_4]}{K_3[CCl_3]}](/tpl/images/0590/9105/a0988.png)
![\text{Rate}=K_2\times \sqrt{K_1[Cl_2]}\times \frac{[CCl_4]}{K_3[CCl_3]}\times [CHCl_3]\\\\\text{Rate}=k[Cl_2]^{1/2}[CCl_4][CHCl_3][CCl_3]^{-1}](/tpl/images/0590/9105/79491.png)


