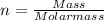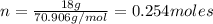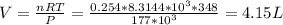Chemistry, 08.04.2020 23:16 Wemaybewrong
If there is 18.0 g of Cl2 gas at a temperature of 348 K and a pressure of 177 kPa, what is the volume of the Cl2 gas?

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:30, jaydenbrock
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 23.06.2019 02:50, agm0102
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3
You know the right answer?
If there is 18.0 g of Cl2 gas at a temperature of 348 K and a pressure of 177 kPa, what is the volum...
Questions in other subjects: