
Chemistry, 08.04.2020 22:29 ayoismeisalex
Given the balanced equation:
ZnSO4 + SrCl2 > SrSO4 + ZnCl2
What number of moles of SrCl2 is consumed when 54 g of ZnCl2 is produced?
a) 0.16 b) 0.3 c) 0.79 d) 1.58 e) 0.4

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, akeemedwards12
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2


Chemistry, 22.06.2019 23:10, RealStephani
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(s o4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 00:50, maddysmall32
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
Given the balanced equation:
ZnSO4 + SrCl2 > SrSO4 + ZnCl2
What number...
ZnSO4 + SrCl2 > SrSO4 + ZnCl2
What number...
Questions in other subjects:





Mathematics, 08.07.2019 21:00


Computers and Technology, 08.07.2019 21:00


Physics, 08.07.2019 21:00

Social Studies, 08.07.2019 21:00

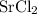 . consumed by 54 grams of zinc chloride has been 0.4 moles. Thus the correct option is e.
. consumed by 54 grams of zinc chloride has been 0.4 moles. Thus the correct option is e.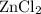 consumes 1 mole of
consumes 1 mole of 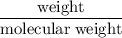
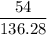 mol
mol

