
Chemistry, 08.04.2020 21:35 charlesiarenee0
Consider the reaction H2(g) + I2(g) <-> HI(g) with an equilibrium constant of 46.3 and a reaction quotient of 525. Which direction will the system shift to?
The equilibrium will shift to the left to favor the reactants.
The equilibrium will shift to the right to favor the products.
The equilibrium will not shift in any direction.
The equilibrium will shift to the forward reaction.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 23.06.2019 01:20, cedricevans41p4j3kx
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
Consider the reaction H2(g) + I2(g) <-> HI(g) with an equilibrium constant of 46.3 and a react...
Questions in other subjects:


Mathematics, 24.07.2019 19:50



Mathematics, 24.07.2019 19:50

Mathematics, 24.07.2019 19:50

Health, 24.07.2019 19:50

Mathematics, 24.07.2019 20:00

Mathematics, 24.07.2019 20:00

History, 24.07.2019 20:00

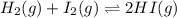
![Q=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0590/2867/236ed.png)


 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored. that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.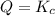 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.

