
When the temperature of a sample of pure water is raised above 25 °C,(1)the hydronium ion concentration will be greater than the hydroxide ion concentration.(2)the hydronium ion concentration will be less than the hydroxide ion concentration.(3)the value of Kw will increase.(4)the hydronium ion concentration could change to 1.0 × 10−10 M.(5)the hydroxide ion concentration could change to 1.0 × 10−10 M.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 04:31, mdarter
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2

Chemistry, 23.06.2019 19:30, lillygon81
Given ch4+2o2—> co2+2h2o what mass of oxygen would be needed to form 17 grams of carbon dioxide
Answers: 1
You know the right answer?
When the temperature of a sample of pure water is raised above 25 °C,(1)the hydronium ion concentrat...
Questions in other subjects:



English, 07.10.2019 16:30

Mathematics, 07.10.2019 16:30


English, 07.10.2019 16:30




Advanced Placement (AP), 07.10.2019 16:30

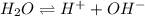
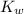 is given by :
is given by :![K_w=[H^+][OH^-]](/tpl/images/0589/8598/bc68a.png)
![K_w\propto [H^+]](/tpl/images/0589/8598/3f687.png)
![K_w\propto [OH^-]](/tpl/images/0589/8598/16555.png)


