
Chemistry, 08.04.2020 19:25 angelaisthebest1700
For the reaction H2 S(g) + I2 (s) ⇌ S(s) + 2 HI(g) Kp = 1.33×10–5 at 333 K. What will be the total pressure of the gases above an equilibrium mixture if, at equilibrium, PHI = 0.010 × PH2 S?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 23.06.2019 01:20, cedricevans41p4j3kx
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
For the reaction H2 S(g) + I2 (s) ⇌ S(s) + 2 HI(g) Kp = 1.33×10–5 at 333 K. What will be the total p...
Questions in other subjects:

History, 27.10.2020 20:10

Mathematics, 27.10.2020 20:10

History, 27.10.2020 20:10

Spanish, 27.10.2020 20:10


Physics, 27.10.2020 20:10

Mathematics, 27.10.2020 20:10


Mathematics, 27.10.2020 20:10

History, 27.10.2020 20:10

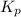
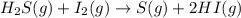
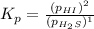
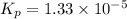
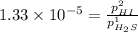
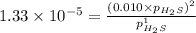
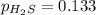
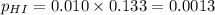
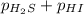 = 0.133+0.0013 = 0.1343 atm
= 0.133+0.0013 = 0.1343 atm

