
Chemistry, 08.04.2020 19:04 vanydparis
Vinegar is a chemical used in cooking, cleaning and other common experiences. A 0.1 M solution of vinegar in water has a [H+] of about 1.3 × 10–3. (You may prefer to think of the hydronium ion concentration, [H3O+], as 1.3 × 10–3.)
A. Write the formula for the calculation of pH, and then show each step as you calculate the pH of a 0.1 M solution of vinegar.
B. Is vinegar an acid or a base? Explain how you know.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 19:10, aamu15
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3
You know the right answer?
Vinegar is a chemical used in cooking, cleaning and other common experiences. A 0.1 M solution of vi...
Questions in other subjects:



Spanish, 02.10.2019 11:10





Mathematics, 02.10.2019 11:10

Biology, 02.10.2019 11:10

Mathematics, 02.10.2019 11:10

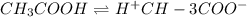
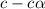
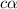
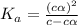
![[H^+]=c\times \alpha](/tpl/images/0589/7489/4fc41.png)
![[H^+]=0.1\times \alpha](/tpl/images/0589/7489/b5870.png)
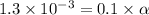
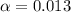
![pH=-log[H^+]](/tpl/images/0589/7489/15713.png)
![pH=-log[1.3\times 10^{-3}]=2.9](/tpl/images/0589/7489/92802.png)


