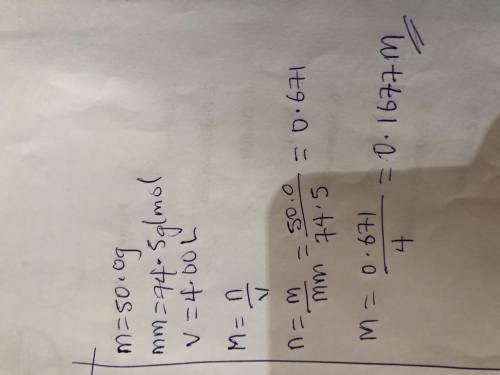50.0 grams of KCl is dissolved in water to make a 4.00 L
solution. What is the molarity of the...

Chemistry, 08.04.2020 19:03 SoccerdudeDylan
50.0 grams of KCl is dissolved in water to make a 4.00 L
solution. What is the molarity of the solution? (Molar mass of
KCl = 74.5 g/mol) ___ M ( Round your answer to the
appropriate number of four significant figures)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 23:10, RealStephani
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(s o4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1

Chemistry, 23.06.2019 01:10, minasotpen1253
Volume is a measurement of how fast particles of a substance are moving
Answers: 3
You know the right answer?
Questions in other subjects:

Mathematics, 31.03.2020 00:12





Mathematics, 31.03.2020 00:12