
Chemistry, 08.04.2020 17:10 DesperatforanA
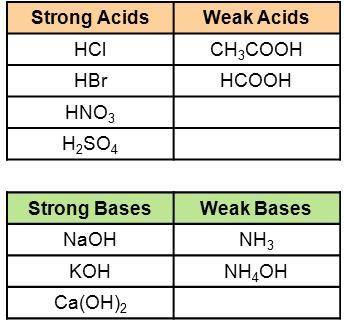
Use the information in the tables to determine whether the solution of the salt formed from the given acid + base combinations will be acidic, basic, or neutral.
HCl + NaOH:
HCl + NH4OH:
CH3COOH + KOH:

Answers: 3


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 03:30, cupcake3103670
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
Use the information in the tables to determine whether the solution of the salt formed from the give...
Questions in other subjects:



Social Studies, 02.11.2020 14:00

Physics, 02.11.2020 14:00

Mathematics, 02.11.2020 14:00

Engineering, 02.11.2020 14:00

Mathematics, 02.11.2020 14:00

Mathematics, 02.11.2020 14:00

Mathematics, 02.11.2020 14:00



