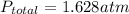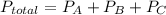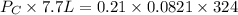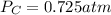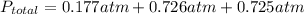Chemistry, 08.04.2020 05:01 hernandezana360
A 7.70 L container holds a mixture of two gases at 51 ° C. The partial pressures of gas A and gas B, respectively, are 0.177 atm and 0.726 atm. If 0.210 mol of a third gas is added with no change in volume or temperature, what will the total pressure become?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 18:20, datboyjulio21
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

You know the right answer?
A 7.70 L container holds a mixture of two gases at 51 ° C. The partial pressures of gas A and gas B,...
Questions in other subjects:

Social Studies, 17.10.2019 11:50

Social Studies, 17.10.2019 11:50

Chemistry, 17.10.2019 11:50

English, 17.10.2019 11:50

Mathematics, 17.10.2019 11:50

French, 17.10.2019 11:50

Arts, 17.10.2019 11:50

Biology, 17.10.2019 11:50