Chemistry, 08.04.2020 05:03 munozjosue258
Chromium has four naturally occurring isotopes. Chromium-50 has a percent abundance of 4.35%, chromium-52 has a percent abundance of 83.79%, chromium-53 has a percent abundance of 9.50%, and chromium-54 has a percent abundance of 2.37%. Based on this information calculate the average atomic mass of chromium. *

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:30, ccispoppin12
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1


Chemistry, 22.06.2019 19:00, elizabethajih99
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
You know the right answer?
Chromium has four naturally occurring isotopes. Chromium-50 has a percent abundance of 4.35%, chromi...
Questions in other subjects:

Mathematics, 30.09.2019 10:30

Business, 30.09.2019 10:30

Biology, 30.09.2019 10:30

Health, 30.09.2019 10:30


Mathematics, 30.09.2019 10:30

Mathematics, 30.09.2019 10:30

Mathematics, 30.09.2019 10:30

Chemistry, 30.09.2019 10:30

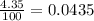
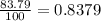
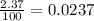
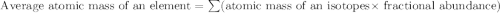
![Z=[(50\times 0.0435)+(52\times 0.8379)+(54\times 0.0237)]](/tpl/images/0589/0146/846f5.png)