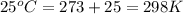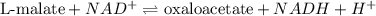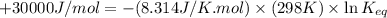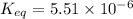Chemistry, 08.04.2020 03:26 pinkmoonlight
In the citric acid cycle, malate is dehydrogenated to oxaloacetate in a highly endergonic reaction with a ΔG’o of +30 kJ mol-1: L‐malate + NAD+ ⇌ oxaloacetate + NADH + H+ Calculate the equilibrium constant K’eq of this reaction. What is the implication of this result?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, girlwholikesanime
Where are each of the three particles located within the atom?
Answers: 1



Chemistry, 22.06.2019 19:20, Lovelybunny321
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1
You know the right answer?
In the citric acid cycle, malate is dehydrogenated to oxaloacetate in a highly endergonic reaction w...
Questions in other subjects:

Health, 31.03.2020 21:17

History, 31.03.2020 21:17


Health, 31.03.2020 21:17

Chemistry, 31.03.2020 21:17



Mathematics, 31.03.2020 21:17


Mathematics, 31.03.2020 21:17

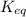 of this reaction is,
of this reaction is, 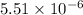
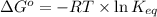
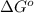 = standard Gibbs free energy = +30 kJ/mol = +30000 J/mol
= standard Gibbs free energy = +30 kJ/mol = +30000 J/mol