
Chemistry, 08.04.2020 03:30 joselaboyNC16
What is the final acetate ion concentration when 69. g Ba(C2H3O2)2 is dissolved in enough water to make 970. mL of solution? The molar mass of Ba(C2H3O2)2 is 255.415 g/mol.
0.28 M
0.64 M
0.91 M
0.20 M
0.56 M

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 21:30, liamgreene90
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
What is the final acetate ion concentration when 69. g Ba(C2H3O2)2 is dissolved in enough water to m...
Questions in other subjects:










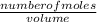
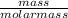
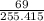 = 0.27moles
= 0.27moles 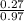 = 0.28M
= 0.28M


