

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, cordovamaria22
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 21.06.2019 22:00, anarosa331hotmailcom
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 21.06.2019 22:30, micvar9646
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3
You know the right answer?
Stock solutions of nitric acid have concentration of 16.0 M. What volume of stock solution is needed...
Questions in other subjects:



English, 22.10.2020 14:01




Social Studies, 22.10.2020 14:01

Mathematics, 22.10.2020 14:01

English, 22.10.2020 14:01

Mathematics, 22.10.2020 14:01

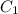 = 16 M
= 16 M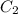 = 2 M
= 2 M = 2 L = 2000 mL
= 2 L = 2000 mL
 x
x  ⇒
⇒ 

