
C6H12O6 (s) + NH3 (g) -> C3H8O3 (l) + C2H6O(l) +CH1.83O0.56N0.17 (s) + CO2(g) + H2O Note that 1 mole glucose produces 0.5 mol glycerol and that the ratio of NH3 to H20 is 1:1. During preparation , you accidentally add too much glucose. However, you decide to let the bioreactor run anyway. After the reaction goes to completion at least one of the starting reactants is used up, you discover that 1.4 lb-m ethanol had been produced. How much heat had been produced and subsequently removed from the system. How much glucose did you initially add to the reactor?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, ajsoccer1705
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 14:30, hjlhdjfhjh
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 23.06.2019 01:00, stefaniethibodeaux
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
C6H12O6 (s) + NH3 (g) -> C3H8O3 (l) + C2H6O(l) +CH1.83O0.56N0.17 (s) + CO2(g) + H2O Note that 1 m...
Questions in other subjects:

Biology, 05.09.2019 05:10


Business, 05.09.2019 05:10



Mathematics, 05.09.2019 05:10




Mathematics, 05.09.2019 05:10

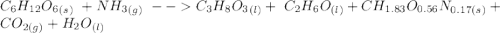
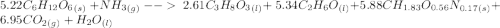
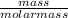
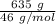
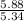 moles of S. cerevisiae formed.
moles of S. cerevisiae formed.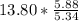 moles of S. cerevisiae formed
moles of S. cerevisiae formed

