
Chegg Aqueous hydrobromic acid will react with solid sodium hydroxide to produce aqueous sodium bromide and liquid water . Suppose 17.0 g of hydrobromic acid is mixed with 14. g of sodium hydroxide. Calculate the minimum mass of hydrobromic acid that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, mauifrifer3986
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 02:30, ijustneedhelp29
Which of the following statements are incorrect?
Answers: 3
You know the right answer?
Chegg Aqueous hydrobromic acid will react with solid sodium hydroxide to produce aqueous sodium brom...
Questions in other subjects:


Chemistry, 20.09.2020 06:01


Chemistry, 20.09.2020 06:01

Biology, 20.09.2020 06:01





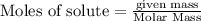
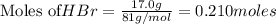
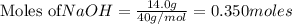

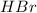 require = 1 mole of
require = 1 mole of 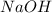
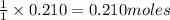 of
of 

