
Chemistry, 08.04.2020 01:44 jayline2003
: Citric acid, H3C6H5O7, is a triprotic acid. Consider a buffer system comprising H2C6H5O7 - and HC6H5O7 2- ions. What is the net ionic equation for the reaction that occurs when NaOH is added to a buffer containing H2C6H5O7 - and HC6H5O7 2- ?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, kaliloabousjbf
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 06:30, khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1
You know the right answer?
: Citric acid, H3C6H5O7, is a triprotic acid. Consider a buffer system comprising H2C6H5O7 - and HC6...
Questions in other subjects:

Mathematics, 01.02.2021 09:10

Engineering, 01.02.2021 09:10


French, 01.02.2021 09:10

Spanish, 01.02.2021 09:10

History, 01.02.2021 09:10



Mathematics, 01.02.2021 09:10

World Languages, 01.02.2021 09:10


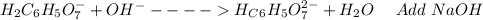
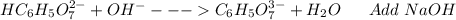
 and
and 



