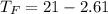Chemistry, 08.04.2020 01:15 brianna8739
Commercial cold packs consist of solid ammonium nitrate and water. NH 4NO 3( s) absorbs 25.69 kJ of heat per mole dissolved in water. In a coffee-cup calorimeter, 3.40 g NH 4NO 3( s) is dissolved in 100.0 g of water at 21.0 °C. What is the final temperature of the solution? Assume that the solution has a specific heat capacity of 4.18 J/g•K.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, sleimanabir
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 21.06.2019 21:30, gallegosarmanni
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 21.06.2019 22:30, dinosaur10
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 06:00, momof7hardings
When would a bouncy ball have the most potential energy
Answers: 2
You know the right answer?
Commercial cold packs consist of solid ammonium nitrate and water. NH 4NO 3( s) absorbs 25.69 kJ of...
Questions in other subjects:

History, 21.09.2021 18:50

Mathematics, 21.09.2021 18:50

English, 21.09.2021 18:50

Mathematics, 21.09.2021 18:50


Mathematics, 21.09.2021 18:50

English, 21.09.2021 18:50


Business, 21.09.2021 18:50

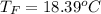
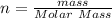
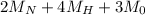
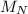 (molar mass of Nitrogen) , 1 for
(molar mass of Nitrogen) , 1 for 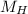 molar mass of hydrogen, 16 for
molar mass of hydrogen, 16 for 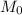 molar mass of oxygen
molar mass of oxygen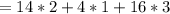
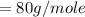
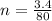
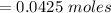

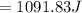
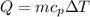
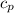 (Specific heat capacity)
(Specific heat capacity) the subject
the subject 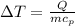
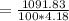
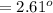 C
C