Chemistry, 08.04.2020 01:03 kenziepickup
A sample of ideal gas is heated in a 2L vessel at a temperature of 320 Kelvin. the pressure in the vessel is 2.5 atm. What is the new pressure in the vessel if the volume is halved and the temperature is reduced to 250 Kelvin?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 06:50, summerjoiner
What is the volume of 3.2 moles of chlorine gas (cl2) at 295 k and 1.1 atm?
Answers: 1

Chemistry, 23.06.2019 07:30, lifeislove3251
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1

Chemistry, 23.06.2019 16:00, michibabiee
Which of the following is a reason to make an armature the parent of a creature
Answers: 1
You know the right answer?
A sample of ideal gas is heated in a 2L vessel at a temperature of 320 Kelvin. the pressure in the v...
Questions in other subjects:




Mathematics, 12.12.2020 16:40

History, 12.12.2020 16:40



Spanish, 12.12.2020 16:40

Computers and Technology, 12.12.2020 16:40

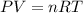
 ..............(1)
..............(1) ................(2)
................(2)