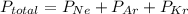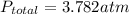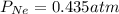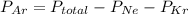Chemistry, 08.04.2020 00:46 isaiahst573
A sealed flask contains Ne, Ar, and Kr gas. If the total pressure in the flask is 3.782 atm, the partial pressure of Ne is 0.435 atm, and the partial pressure of Kr is 1.613 atm, what is the partial pressure of Ar

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, shradhwaip2426
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3



Chemistry, 22.06.2019 10:00, valdezlizbeth6652
Why is carbon ideal for making different compounds?
Answers: 2
You know the right answer?
A sealed flask contains Ne, Ar, and Kr gas. If the total pressure in the flask is 3.782 atm, the par...
Questions in other subjects:





Geography, 27.01.2020 17:31



French, 27.01.2020 17:31


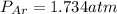 = partial pressure of Ar
= partial pressure of Ar