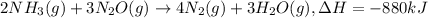When 2 moles of NH3(g) react with N2O(g) to form N2(g) and H2O(g) according to the following equation, 880 kJ of energy are evolved. 2NH3(g) + 3N2O(g)4N2(g) + 3H2O(g) Is this reaction endothermic or exothermic? What is the value of q? kJ An error has been detected in your answer. Che

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1


Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

You know the right answer?
When 2 moles of NH3(g) react with N2O(g) to form N2(g) and H2O(g) according to the following equatio...
Questions in other subjects:


Mathematics, 26.09.2019 19:30

History, 26.09.2019 19:30

History, 26.09.2019 19:30

Geography, 26.09.2019 19:30




Mathematics, 26.09.2019 19:30

 comes out to be negative.
comes out to be negative.