Chemistry, 07.04.2020 23:39 crystalclear99
Select the true statements.
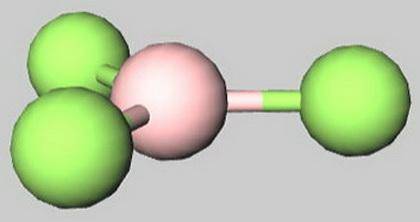
A) BF 3 has a trigonal planar shape.
B) Molecules with three outer atoms always have a trigonal planar shape.
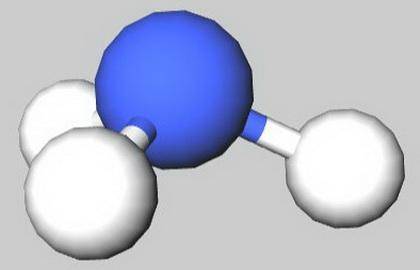
C) NH 3 has a trigonal planar shape.
D) Trigonal planar molecules have three regions of high-electron density around the central at

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 18:20, datboyjulio21
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 01:30, kayleg907436
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 01:40, georgehall3027
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2
You know the right answer?
Select the true statements.
A) BF 3 has a trigonal planar shape.
B) Molecules wi...
A) BF 3 has a trigonal planar shape.
B) Molecules wi...
Questions in other subjects: