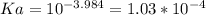Chemistry, 07.04.2020 23:52 brendaesme
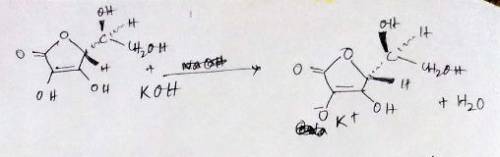
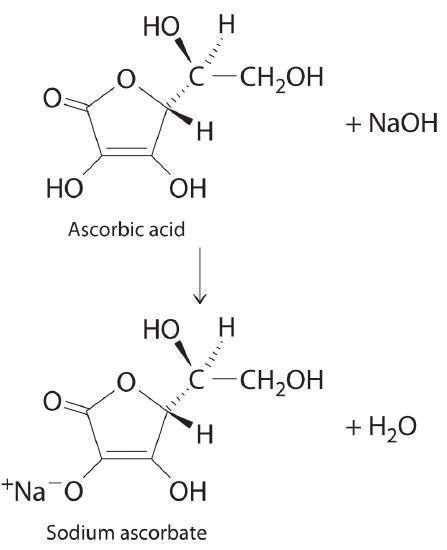
A 0.552-g sample of ascorbic acid was dissolved in water to a total volume of 0.20 mL and titrated with 0.1103 M KOH. The equivalence point occurred at 28.42 mL. The pH of the solution at 10.0mL of added base was 3.72. From this data, determine the molar rmass and Ka for vitamin C.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:10, leo4687
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2


Chemistry, 22.06.2019 18:30, kate3887
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
A 0.552-g sample of ascorbic acid was dissolved in water to a total volume of 0.20 mL and titrated w...
Questions in other subjects:


Mathematics, 10.01.2020 21:31

Mathematics, 10.01.2020 21:31

Mathematics, 10.01.2020 21:31

Mathematics, 10.01.2020 21:31

Mathematics, 10.01.2020 21:31


Chemistry, 10.01.2020 21:31

History, 10.01.2020 21:31