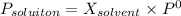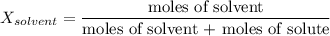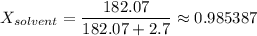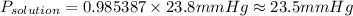This is Raoult law & vapor pressure problem: high school
2.7 moles of Tang are added...

This is Raoult law & vapor pressure problem: high school
2.7 moles of Tang are added to a pitcher containing 3.28 liters of H2O at a temperature of 25°C. The vapor pressure of water alone is 23.8 mm Hg at 25o C. What is the new vapor pressure of the Tang solution?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, orlando19882000
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 23.06.2019 00:20, HernanJe6
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 03:40, ElegantEmerald
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
Questions in other subjects:





Mathematics, 12.11.2020 01:00

Chemistry, 12.11.2020 01:00