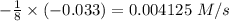Chemistry, 03.12.2019 16:31 madison1284
Consider the reaction: 8h2s(g)+4o2(g)→8h2o(g)+s8(g). δ[h2s]/δt = -0.033m/s. find δ[o2]/δt. δ[h2o]/δt. δ[s8]/δt. find the rate of the reaction.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, anthony4034
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 08:40, jeffcarpenter
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1


Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
Consider the reaction: 8h2s(g)+4o2(g)→8h2o(g)+s8(g). δ[h2s]/δt = -0.033m/s. find δ[o2]/δt. δ[h2o]/δ...
Questions in other subjects:





Mathematics, 19.04.2021 18:40

Mathematics, 19.04.2021 18:40

Mathematics, 19.04.2021 18:40


Geography, 19.04.2021 18:40

Mathematics, 19.04.2021 18:40

![\frac{[\Delta O_2]}{\Delta t} = -0.0165\ M](/tpl/images/0401/1414/d412a.png)
![\frac{[\Delta H_2O]}{\Delta t}= 0.033\ M/s](/tpl/images/0401/1414/ccd80.png)
![\frac{[\Delta S_8]}{\Delta t} = 0.004125\ M/s](/tpl/images/0401/1414/d55d1.png)
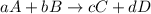
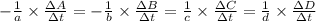
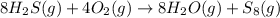
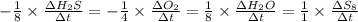
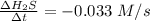
![-\frac{1}{8}\times \frac{[\Delta H_2S]}{\Delta t} =-\frac{1}{4}\times \frac{[\Delta O_2]}{\Delta t}](/tpl/images/0401/1414/fd6a9.png)
![-\frac{1}{8}\times (-0.33) =-\frac{1}{4}\times \frac{[\Delta O_2]}{\Delta t}](/tpl/images/0401/1414/02deb.png)
![-\frac{[\Delta O_2]}{\Delta t} = \frac{4}{8} \times (0.033) = 0.0165\ M](/tpl/images/0401/1414/323db.png)
![\frac{[\Delta O_2]}{\Delta t} = -0.0165\ M/s](/tpl/images/0401/1414/d516e.png)
![-\frac{1}{8}\times \frac{[\Delta H_2S]}{\Delta t} =\frac{1}{8}\times \frac{[\Delta H_2O]}{\Delta t}](/tpl/images/0401/1414/d13b5.png)
![\frac{[\Delta H_2O]}{\Delta t}=-\frac{8}{8}\times \frac{[\Delta H_2S]}{\Delta t}](/tpl/images/0401/1414/4e162.png)
![\frac{[\Delta H_2O]}{\Delta t}=-\frac{8}{8}\times (-0.033)](/tpl/images/0401/1414/b29cf.png)
![-\frac{1}{8}\times \frac{[\Delta H_2S]}{\Delta t} =\frac{1}{1}\times \frac{[\Delta S_8]}{\Delta t}](/tpl/images/0401/1414/1cad1.png)
![\frac{[\Delta S_8]}{\Delta t}=-\frac{1}{8}\times (-0.033)=0.004125\ M/s](/tpl/images/0401/1414/4b7e9.png)
![-\frac{1}{8}\times \frac{[\Delta H_2S]}{\Delta t}](/tpl/images/0401/1414/78436.png)