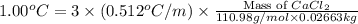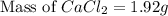Chemistry, 07.04.2020 23:30 mbprez6029
To be able to see a measurable effect, your boiling point elevation must be at least 1.00°C. Knowing that the Kb of water is 0.512\frac{^\circ\text{C}\cdot\text {kg}}{\text{mol}}0.512 ∘ C ⋅ kg mol, determine what mass of calcium chloride (in g) is needed to see at least a 1.00^\circ\text{C}1.00 ∘ C boiling point increase in 26.63 mL of water.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, board1692
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3



Chemistry, 22.06.2019 21:00, rhondafits9000
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
To be able to see a measurable effect, your boiling point elevation must be at least 1.00°C. Knowing...
Questions in other subjects:


Chemistry, 09.09.2020 04:01





Biology, 09.09.2020 04:01


Geography, 09.09.2020 04:01

Mathematics, 09.09.2020 04:01

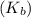 for water =
for water = 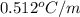
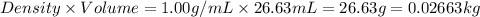
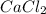 = 110.98 g/mole
= 110.98 g/mole
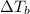 = change in boiling point =
= change in boiling point = 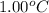
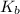 = boiling point constant for water
= boiling point constant for water