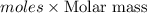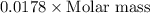Chemistry, 07.04.2020 22:58 alyssahomeworkneeds
Given the following information: 1.6 g of an unknown monoprotic acid (HA) required 50.80 mL of a 0.35 M NaOH solution to reach the equivalence point, calculate the molar mass (g/mol) of the acid. Enter the value ONLY. Do not include the units.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2


Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2
You know the right answer?
Given the following information: 1.6 g of an unknown monoprotic acid (HA) required 50.80 mL of a 0.3...
Questions in other subjects:

Mathematics, 19.12.2020 19:50


Mathematics, 19.12.2020 20:00


Mathematics, 19.12.2020 20:00


Spanish, 19.12.2020 20:00

Mathematics, 19.12.2020 20:00

English, 19.12.2020 20:00

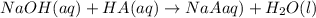
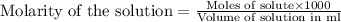 .....(1)
.....(1)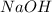 solution = 0.35 M
solution = 0.35 M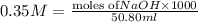
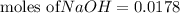
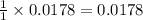 moles of HCl
moles of HCl