
Chemistry, 07.04.2020 22:38 JocelynC24
A certain amount of gas at 18.0°C and at a pressure of 0.900 atm is contained in a glass vessel. Suppose that the vessel can withstand a pressure of 1.90 atm. How high can you raise the temperature of the gas without bursting the vessel? In other words, at what temperature will the glass vessel shatter, in degrees Celsius.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, sillslola816oxb5h7
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 22.06.2019 02:20, ayoismeisalex
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 04:00, heavyhearttim
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 14:30, belindajolete
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2
You know the right answer?
A certain amount of gas at 18.0°C and at a pressure of 0.900 atm is contained in a glass vessel. Sup...
Questions in other subjects:



Mathematics, 25.09.2019 11:30

Mathematics, 25.09.2019 11:30

Mathematics, 25.09.2019 11:30




Social Studies, 25.09.2019 11:30

 = 18 °c = 291 K
= 18 °c = 291 K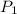 = 0.9 atm
= 0.9 atm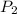 = 1.9 atm
= 1.9 atm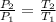
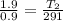
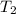 = 613.33 K = 341.33°c
= 613.33 K = 341.33°c


