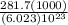Chemistry, 07.04.2020 22:28 mmassaro19
It takes 281.7 kJ of energy to remove 1 mole of electrons from the atoms on the surface of lithium metal. If lithium metal is irradiated with 261-nm light, what is the maximum kinetic energy the released electrons can have

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:10, YatesDevon3371
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 00:10, scottbrandon653
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
It takes 281.7 kJ of energy to remove 1 mole of electrons from the atoms on the surface of lithium m...
Questions in other subjects:

Mathematics, 12.05.2021 08:40

History, 12.05.2021 08:40


Business, 12.05.2021 08:40

Mathematics, 12.05.2021 08:40

History, 12.05.2021 08:40

Social Studies, 12.05.2021 08:40



 Joule
Joule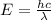 ------------ (1)
------------ (1) J s
J s
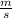
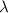 = 261 nm = 261 ×
= 261 nm = 261 × 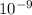 m
m