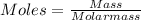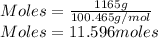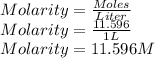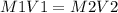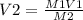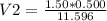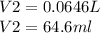Chemistry, 08.04.2020 00:19 rhineharttori
A sample of commercial perchloric acid is 70.0% HClO4 by mass; its density is 1.664 g/mL. How many milliliters of this concentrated HClO4 would be required to prepare 500. mL of 1.50 M HClO4 solution?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 23.06.2019 01:30, emfranco1
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1

Chemistry, 23.06.2019 04:00, Tiredd7838
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1

Chemistry, 23.06.2019 08:00, codybrocs9624
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 2
You know the right answer?
A sample of commercial perchloric acid is 70.0% HClO4 by mass; its density is 1.664 g/mL. How many m...
Questions in other subjects:

Biology, 28.01.2020 03:31

History, 28.01.2020 03:31


Mathematics, 28.01.2020 03:31


History, 28.01.2020 03:31




History, 28.01.2020 03:31