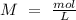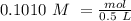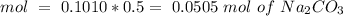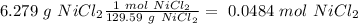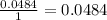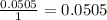Chemistry, 07.04.2020 21:28 danielahalesp87vj0
Determine the limiting reactant for the reaction of sodium carbonate and nickel(II) chloride using the quantities listed below. 6.279 g solid nickel(II) chloride 500.0 mL of 0.1010 M sodium carbonate

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
Determine the limiting reactant for the reaction of sodium carbonate and nickel(II) chloride using t...
Questions in other subjects:

Mathematics, 13.01.2021 16:10


Social Studies, 13.01.2021 16:10

Mathematics, 13.01.2021 16:10


Mathematics, 13.01.2021 16:10


Geography, 13.01.2021 16:10

Business, 13.01.2021 16:10

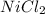
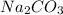 ) and the Nickel (II) Chloride (
) and the Nickel (II) Chloride (