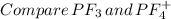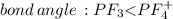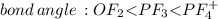Chemistry, 07.04.2020 21:43 ginger1234
Place the following in order of increasing F-A-F bond angle, where A represents the central atom in each molecule. PF3 OF2 PF4
a. PF3 < OF2 < PF4
b. OF2 < PF3 < PF4
c. PF4 < OF2 < PF3
d. PF4 < PF3 < OF2
e. OF2 < PF4 < PF3
f. PF3 < PF4 < OF2

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, kichensides
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1


Chemistry, 22.06.2019 12:00, Alexislol7908
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
Place the following in order of increasing F-A-F bond angle, where A represents the central atom in...
Questions in other subjects:

Biology, 07.05.2021 08:50

Chemistry, 07.05.2021 08:50


Mathematics, 07.05.2021 08:50

Biology, 07.05.2021 08:50



Mathematics, 07.05.2021 08:50


Mathematics, 07.05.2021 08:50

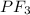 with
with 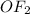
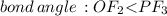
 does no exist it exist only in the form of
does no exist it exist only in the form of 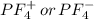 and here assuming
and here assuming 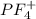 because in case of
because in case of 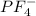 difficult to compare bond angle because here two different type of bond angle is present.
difficult to compare bond angle because here two different type of bond angle is present.