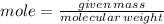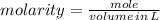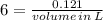To produce 40.0 g of silver chromate, you will need at least 23.4 g of potassium chromate in solution as a reactant. All you have on hand in the stock room is 5 L of a 6.00 M K2CrO4 solution. What volume of the solution is needed to give you the 23.4 g K2CrO4 needed for the reaction?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bobbycisar1205
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2

Chemistry, 22.06.2019 21:30, leenzazou587
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
To produce 40.0 g of silver chromate, you will need at least 23.4 g of potassium chromate in solutio...
Questions in other subjects:


Mathematics, 15.11.2019 05:31

Mathematics, 15.11.2019 05:31



Mathematics, 15.11.2019 05:31




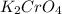 required=20.1 ml
required=20.1 ml