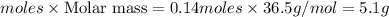Chemistry, 07.04.2020 21:05 laura52677
G Aqueous hydrochloric acid will react with solid sodium hydroxide to produce aqueous sodium chloride and liquid water . Suppose 20. g of hydrochloric acid is mixed with 16.3 g of sodium hydroxide. Calculate the minimum mass of hydrochloric acid that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, janetexcoelho
What does the mass of 0.7891 mol of ferric oxide (fe2o3)
Answers: 1


Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
G Aqueous hydrochloric acid will react with solid sodium hydroxide to produce aqueous sodium chlorid...
Questions in other subjects:


History, 24.06.2019 15:00

English, 24.06.2019 15:00





English, 24.06.2019 15:00


SAT, 24.06.2019 15:00

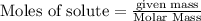
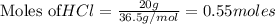
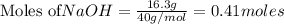
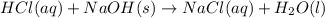
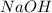 require = 1 mole of
require = 1 mole of 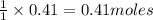 of
of 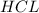 is the excess reagent.
is the excess reagent.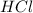 left =
left =