
Chemistry, 07.04.2020 19:41 BaileyRyan8320
A sample of pure NH4HS is placed in a sealed 2.0-L container and heated to 550 K at which the equilibrium constant is 3.5 x 10-3. Once the reaction reaches equilibrium, what mass of NH3 is present in the container

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:30, abdullaketbi71
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 23:00, ceejay8005
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 08:30, zhjzjzzj8225
Explain how to convert from one unit to another in the metric system.
Answers: 3
You know the right answer?
A sample of pure NH4HS is placed in a sealed 2.0-L container and heated to 550 K at which the equili...
Questions in other subjects:

Mathematics, 29.10.2020 22:40

Arts, 29.10.2020 22:40


Advanced Placement (AP), 29.10.2020 22:40



Mathematics, 29.10.2020 22:40


History, 29.10.2020 22:40

 in the container is 2.074 gram
in the container is 2.074 gram
 lit
lit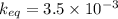

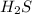 is formed.
is formed.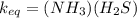

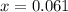 M
M
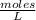
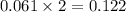 moles.
moles. g
g

