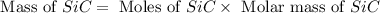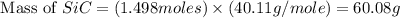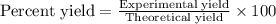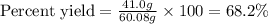Equation: SiO2 + 3C = SiC + 2CO When 90.0 g of silicon dioxide is heated with an excess of carbon, 41.0 g of silicon carbide is produced. What is the percent yield of this reaction? (find the theoretical amount of SiC using stoichiometry, then calculate percent yield)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, sophiapknight
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3

Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3
You know the right answer?
Equation: SiO2 + 3C = SiC + 2CO When 90.0 g of silicon dioxide is heated with an excess of carbon, 4...
Questions in other subjects:


Mathematics, 20.08.2020 20:01

Computers and Technology, 20.08.2020 20:01


History, 20.08.2020 20:01

English, 20.08.2020 20:01




Mathematics, 20.08.2020 20:01

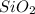 = 90.0 g
= 90.0 g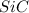 = 41.0 g
= 41.0 g
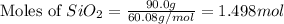
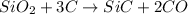
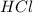 react to give 1.498 mole of
react to give 1.498 mole of