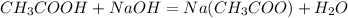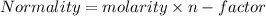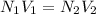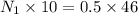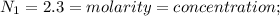Chemistry, 07.04.2020 19:22 genyjoannerubiera
In a laboratory experiment, a student titrates 10.0 mL of acetic acid, CH3COOH, solution with 0.5 M NaOH. The endpoint was reached when 46.00 mL of 0.50 M NaOH were delivery. What was the concentration of acetic acid in the solution

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3


Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 15:30, neariah24
Plz me ! 1 which of earths spheres contains most of its mass? a atmosphere b hydrosphere c geosphere* d biosphere 2 erosion and weathering are examples of which types of forces? a constructive forces b destructive forces* c gravitational forces d inertia-related forces 3 which of the following statements about earths atmosphere is true? a earths atmosphere contains 78% water vapor which is essentail to life b earths atmosphere contains 21% oxygen c earths atmosphere contains carbon dioxide which all life forms require d earths atmosphere allows radiation from the sun to pass through it and warm earths surface* 4 the strenght of the force of gravity between two objects is determined by which of the following factors? select all that apply a the messes of the objects* b the distance between the objects* c the volumes of the objects d the surface area of the objects 5 earth and moon are kept in there respective orbits due to the influence of a inertia b gravity c gravity and inertia* d neither gravity or inertia if you answer all questions right i will give
Answers: 1
You know the right answer?
In a laboratory experiment, a student titrates 10.0 mL of acetic acid, CH3COOH, solution with 0.5 M...
Questions in other subjects:









Mathematics, 05.09.2020 05:01