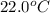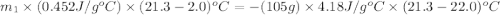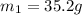Chemistry, 07.04.2020 19:02 precioushayhay
105 mL of H2O is initially at room temperature (22.0∘C). A chilled steel rod at 2.0∘C is placed in the water. If the final temperature of the system is 21.3 ∘C, what is the mass of the steel bar? Specific heat of water = 4.18 J/g⋅∘C Specific heat of steel = 0.452 J/g⋅∘C

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, kingamir
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1

Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 23.06.2019 03:00, amberskids2
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2
You know the right answer?
105 mL of H2O is initially at room temperature (22.0∘C). A chilled steel rod at 2.0∘C is placed in t...
Questions in other subjects:


English, 30.01.2021 02:30

Mathematics, 30.01.2021 02:30


Social Studies, 30.01.2021 02:30

Mathematics, 30.01.2021 02:30

Mathematics, 30.01.2021 02:30



Mathematics, 30.01.2021 02:30

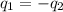
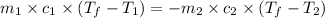
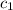 = specific heat of steel =
= specific heat of steel = 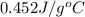
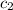 = specific heat of water =
= specific heat of water = 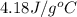
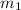 = mass of steel rod = ?
= mass of steel rod = ?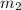 = mass of water =
= mass of water = 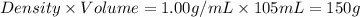
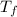 = final temperature of mixture =
= final temperature of mixture = 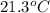
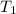 = initial temperature of steel =
= initial temperature of steel = 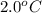
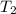 = initial temperature of water =
= initial temperature of water =