
Chemistry, 07.04.2020 16:54 jagarcia2024
Calculate the [ OH − ] and the pH of a solution with an [ H + ] = 0.090 M at 25 °C . [ OH − ] = M pH = Calculate the [ H + ] and the pH of a solution with an [ OH − ] = 0.00098 M at 25 °C . [ H + ] = M pH = Calculate the [ H + ] and the [ OH − ] of a solution with a pH = 10.15 at 25 °C . [ H + ] = M [ OH − ] =

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1


You know the right answer?
Calculate the [ OH − ] and the pH of a solution with an [ H + ] = 0.090 M at 25 °C . [ OH − ] = M pH...
Questions in other subjects:


Biology, 22.10.2020 07:01




Mathematics, 22.10.2020 07:01




Biology, 22.10.2020 07:01

![[OH^-]=1.09\times 10^{-13}](/tpl/images/0586/5079/12eb0.png) and pH = 1.04
and pH = 1.04![[H^+]=1.02\times 10^{-11}](/tpl/images/0586/5079/f4659.png) and
and 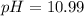
![[H^+]=7.08\times 10^{-11}](/tpl/images/0586/5079/77629.png) and
and ![[OH^-]=1.41\times 10^{-4}](/tpl/images/0586/5079/9fe1b.png)
![pH=-\log [H^+]](/tpl/images/0586/5079/37e81.png)
![pOH=-\log [OH^-]](/tpl/images/0586/5079/1fac1.png)
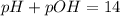
![[H^+]=0.090M](/tpl/images/0586/5079/6c0fa.png)
![pH=-\log [0.090]=1.04](/tpl/images/0586/5079/718ea.png)
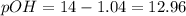
![12.96=-log[OH^-]](/tpl/images/0586/5079/9dc6e.png)
![[OH^-]=0.00098M](/tpl/images/0586/5079/a32b5.png)
![pOH=-\log [0.00098]=3.01](/tpl/images/0586/5079/735b6.png)
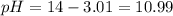
![10.99=-log[H^+]](/tpl/images/0586/5079/318f1.png)
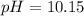
![10.15=-\log [H^+]](/tpl/images/0586/5079/097ad.png)
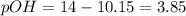
![3.85=-log[OH^-]](/tpl/images/0586/5079/b9fea.png)


