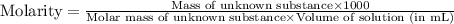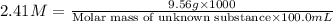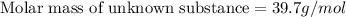Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, katherineweightman
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 21:00, itasykamila
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 21:20, 50057543
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
A chemical test has determined the concentration of a solution of an unknown substance to be 2.41 M....
Questions in other subjects:




English, 25.12.2019 15:31

World Languages, 25.12.2019 15:31



Mathematics, 25.12.2019 15:31

Mathematics, 25.12.2019 15:31