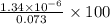Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 23.06.2019 01:00, jazzy200232
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 02:30, elyzarobertson
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 03:00, jaidencoolman2866
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
Solid NaBr is slowly added to a solution that is 0.073 M in Cu+ and 0.073 M in Ag+.Which compound wi...
Questions in other subjects:

Biology, 31.07.2019 09:40




Social Studies, 31.07.2019 09:40




History, 31.07.2019 09:40

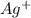 when CuBr just begins to precipitate is,
when CuBr just begins to precipitate is, 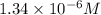
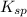 for CuBr is
for CuBr is 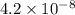
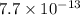
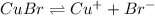
![K_{sp}=[Cu^+][Br^-]](/tpl/images/0586/4611/12867.png)
![4.2\times 10^{-8}=0.073\times [Br^-]](/tpl/images/0586/4611/29ab2.png)
![[Br^-]=5.75\times 10^{-7}M](/tpl/images/0586/4611/05d81.png)
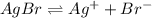
![K_{sp}=[Ag^+][Br^-]](/tpl/images/0586/4611/90aa5.png)
![7.7\times 10^{-13}=[Ag^+]\times 5.75\times 10^{-7}M](/tpl/images/0586/4611/053c9.png)
![[Ag^+]=1.34\times 10^{-6}M](/tpl/images/0586/4611/7e622.png)