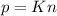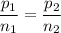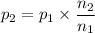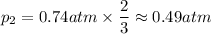A gas exerts a pressure of 0.74 atm in a certain container. Suddenly, a chemical change occurs that consumes half of the molecules originally present and forms two new molecules for every three consumed. Determine the new pressure in the container if the volume of the container and the temperature are unchanged. The reaction that occurs is: 3A(g) —> 2B(g)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, psychocatgirl1
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone, due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 20:00, ahnorthcutt4965
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
A gas exerts a pressure of 0.74 atm in a certain container. Suddenly, a chemical change occurs that...
Questions in other subjects:


History, 12.11.2020 03:10

Chemistry, 12.11.2020 03:10


Spanish, 12.11.2020 03:10

Biology, 12.11.2020 03:10




Health, 12.11.2020 03:10