Chemistry, 07.04.2020 15:52 boweytom6217
Zinc reacts with hydrochloric acid according to the reaction equation Zn ( s ) + 2 HCl ( aq ) ⟶ ZnCl 2 ( aq ) + H 2 ( g ) How many milliliters of 6.50 M HCl ( aq ) are required to react with 2.55 g Zn ( s ) ?

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:30, neidaq12345
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

You know the right answer?
Zinc reacts with hydrochloric acid according to the reaction equation Zn ( s ) + 2 HCl ( aq ) ⟶ ZnCl...
Questions in other subjects:




History, 01.07.2019 02:30

Mathematics, 01.07.2019 02:30



Mathematics, 01.07.2019 02:30


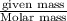

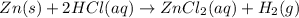
 moles of HCl
moles of HCl .....(1)
.....(1)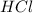 solution = 6.50 M
solution = 6.50 M